![SOLVED: [CoCl4]^2- is a Co^2+ tetrahedral comblex that is colored. 4.2. Explain why this complex is so brightly colored. Complete the sketch of the molecular orbital diagram 4 p≡ provided by adding SOLVED: [CoCl4]^2- is a Co^2+ tetrahedral comblex that is colored. 4.2. Explain why this complex is so brightly colored. Complete the sketch of the molecular orbital diagram 4 p≡ provided by adding](https://cdn.numerade.com/ask_images/a8ab43fa57554054b4968f6b29691ccb.jpg)
SOLVED: [CoCl4]^2- is a Co^2+ tetrahedral comblex that is colored. 4.2. Explain why this complex is so brightly colored. Complete the sketch of the molecular orbital diagram 4 p≡ provided by adding
Predict the number of unpaired electrons in [COCl4]^2- ion on the basis of VBT. [COCl4]^2- - Sarthaks eConnect | Largest Online Education Community
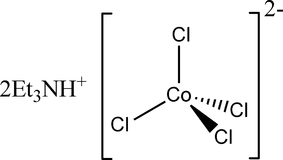
The complex salt $CoCl_{ 4 }^{ 2- }$ has a tetrahedral structure. How many d-electrons are on the cobalt?

Describe the distribution of d electrons in the (CoCl_4)^2- ion. The ion has a tetrahedral geometry. Assume a high-spin complex. | Homework.Study.com

Cobalt transition metal Chemistry cobalt(II)Co2+ complex ions stabilised ligand substitution cobalt(III) Co3+ complexes redox chemical reactions +2 +3 principal oxidation states balanced equations Rhodium Iridium Meitnerium GCE AS A2 IB A level
![Determine the structure and magnetic behaviour of [CoCl4 ]²- using valence bond theory. according to board - Brainly.in Determine the structure and magnetic behaviour of [CoCl4 ]²- using valence bond theory. according to board - Brainly.in](https://hi-static.z-dn.net/files/dbc/4e4b8ae040fa0c9d9e6932ef4496ad25.jpg)
Determine the structure and magnetic behaviour of [CoCl4 ]²- using valence bond theory. according to board - Brainly.in
![Cobalt tetrachloride [CoCl4]2- ion - Structure - hybridization - color - leChatelier principle - YouTube Cobalt tetrachloride [CoCl4]2- ion - Structure - hybridization - color - leChatelier principle - YouTube](https://i.ytimg.com/vi/TBGsBJMN9fU/sddefault.jpg)
Cobalt tetrachloride [CoCl4]2- ion - Structure - hybridization - color - leChatelier principle - YouTube
![SOLVED: What is the correct formula for sodium tetrachlorocobaltate(I)? a. NaZ[CoCl4] b. Na2[CoCl4] c. Na4[CoCl4] d. Na[CoCl4] e. Na[CoCl4] SOLVED: What is the correct formula for sodium tetrachlorocobaltate(I)? a. NaZ[CoCl4] b. Na2[CoCl4] c. Na4[CoCl4] d. Na[CoCl4] e. Na[CoCl4]](https://cdn.numerade.com/ask_previews/9d18200b-36c3-4d2c-904b-4866aaaff2ba_large.jpg)

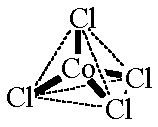
![Predict the hybridisation and geometry of `[CoCl_(4)]^(2-) and [Co(CN)_(4)]^(2-)` Predict the hybridisation and geometry of `[CoCl_(4)]^(2-) and [Co(CN)_(4)]^(2-)`](https://i.ytimg.com/vi/m7yduxa5Ysw/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLDioezo6yafUtFRdVQZgUlgZXiOWg)
![SOLVED: State the nomenclature of (NH4)2[CoCl4] SOLVED: State the nomenclature of (NH4)2[CoCl4]](https://cdn.numerade.com/ask_previews/139d716c-5ff4-455b-a952-0f605374158a_large.jpg)
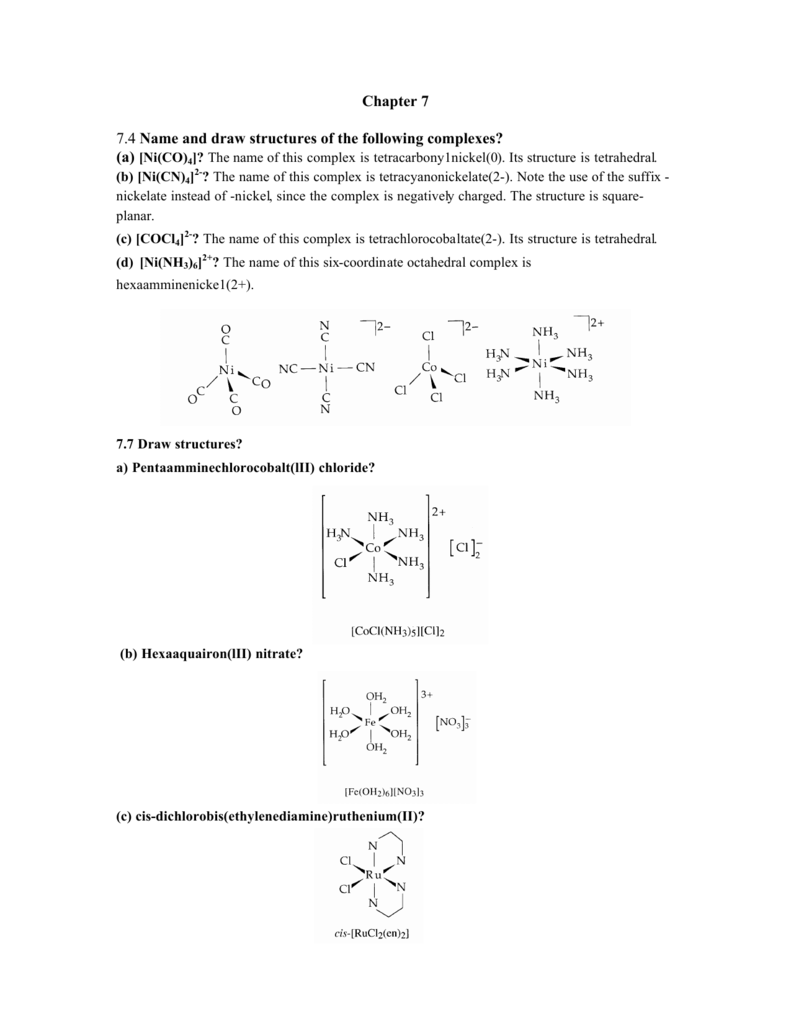
![Write the IUPAC name and hybridisation of K(2)[CoCl(4)(en)] Write the IUPAC name and hybridisation of K(2)[CoCl(4)(en)]](https://static.doubtnut.com/ss/web/1312549.webp)

![The CFSE value of tetrahedral [CoCl4]2 complex is: The CFSE value of tetrahedral [CoCl4]2 complex is:](https://df0b18phdhzpx.cloudfront.net/ckeditor_assets/pictures/1444484/original_22.png)
![Predict the hybridisation and geometry of [CoCl(4)]^(2-) and [Co(CN)(4 Predict the hybridisation and geometry of [CoCl(4)]^(2-) and [Co(CN)(4](https://d10lpgp6xz60nq.cloudfront.net/physics_images/AAK_T5_CHE_C19_E01_010_S02.png)
![Solved Question 13 What is the IUPAC name for K2[CoCl4]? A. | Chegg.com Solved Question 13 What is the IUPAC name for K2[CoCl4]? A. | Chegg.com](https://media.cheggcdn.com/study/804/804d85ef-b8b9-4d52-9504-6c7c40d3bef1/image.png)
![Determine the structure and magnetic behaviour of [CoCl4]2− using valenc.. Determine the structure and magnetic behaviour of [CoCl4]2− using valenc..](https://classroom-images.cdn.askfilo.com/classroom/1675440977482_fsysnizc_3636371.jpg)
![Odia] Write the IUPAC name of the followings. K2 [ CoCl4] Odia] Write the IUPAC name of the followings. K2 [ CoCl4]](https://static.doubtnut.com/ss/web/10807229.webp)