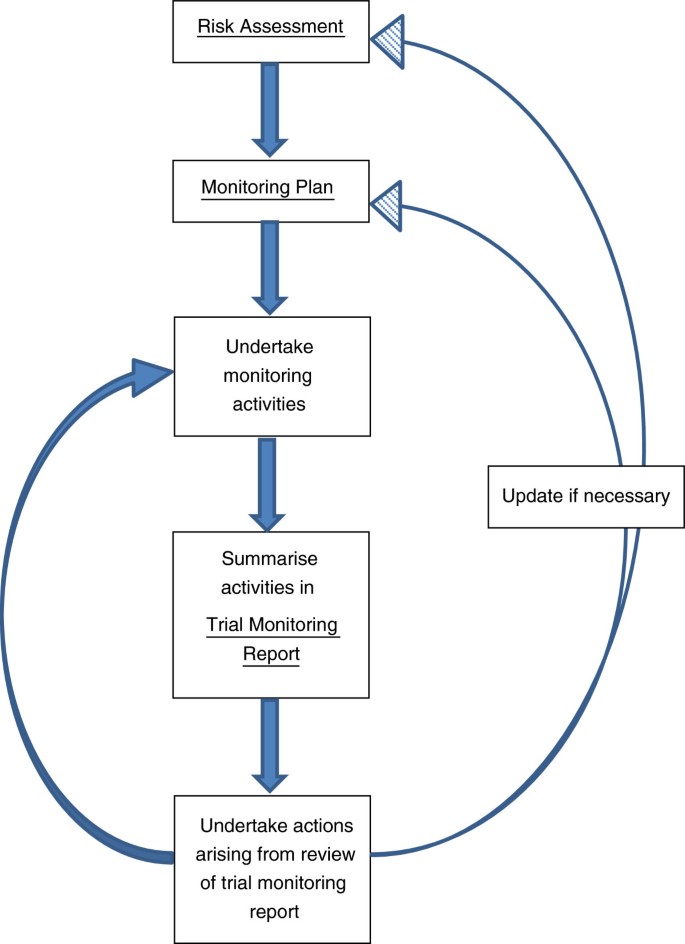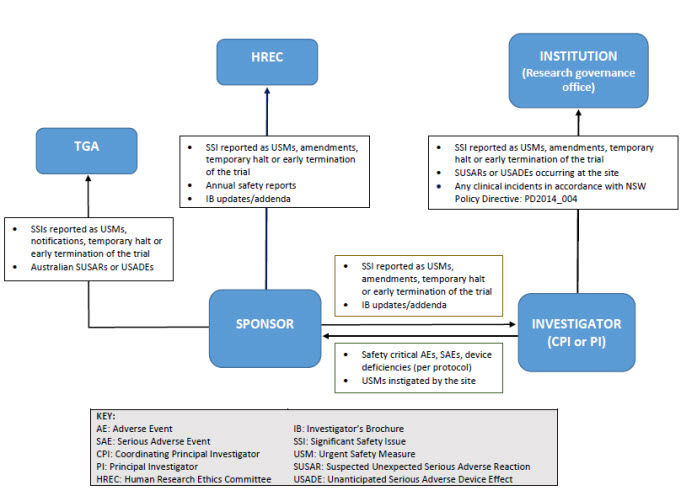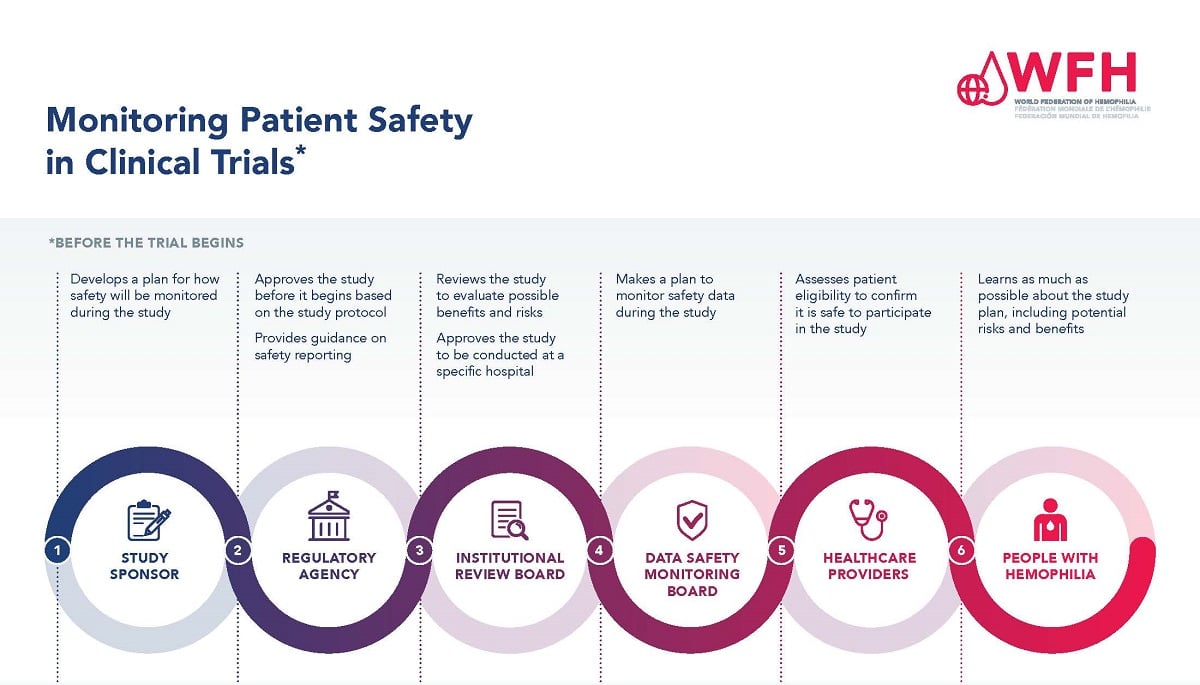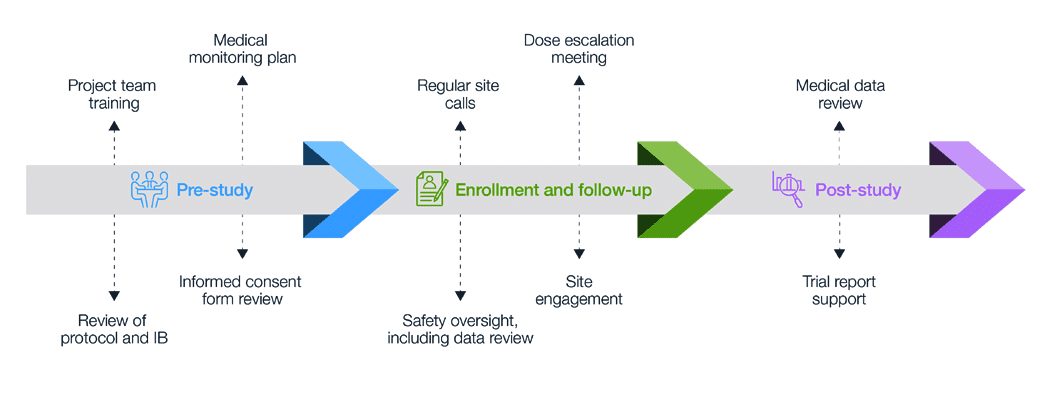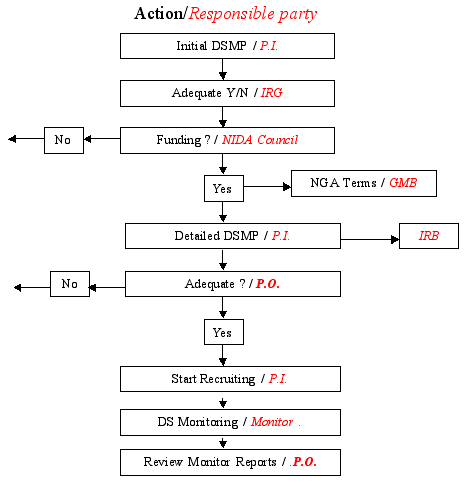
Guidelines for Developing a Data and Safety Monitoring Plan | National Institute on Drug Abuse (NIDA)

Safety Monitoring and Adverse Event Reporting in Clinical Trials: Regulatory Requirements and Best Practices – ParadigmIT

Data Safety and Monitoring Boards Should Be Required for Both Early- and Late-Phase Clinical Trials - ScienceDirect